An international research team including scientists from the Jagiellonian University has published the results of a ground-breaking study on RagAB protein complex, which allows the transport of peptides inside the cells of Porphyromonas gingivalis bacteria, the main factor responsible for the development of periodontal disease. This is the first such system of peptide transport in Gram-negative bacteria to have been described so far. The discovery can lead to the development of medications hindering the growth of these pathogens.
Porphyromonas gingivalis is the main pathogen responsible for periodontal disease – one of the most common human inflammatory diseases. It has also been proven that these bacteria play a role in the development of such conditions as rheumatoid arthritis, cardiovascular diseases, or Alzheimer’s disease. As P. gingivalis cannot use sugars as a source of food, it feeds on the host’s proteins (for instance albumins from blood plasma). They are, however, too big to be absorbed by the bacterium, so P. gingivalis cuts them into smaller chunks (peptides) with enzymes known as gingipains, acting like molecular scissors. Gingipains are attached to the outer membrane of the bacteria, but they can also be released to the extracellular space and thus extend their effective range. In spite of the peptides’ crucial importance for the survival of the bacteria, the way how they manage to get through the outer membrane into the cells so far remained a mystery.
RagAB? Access granted.
The authors the paper “Structural and functional insights into oligopeptide acquisition by the RagAB transporter from Porphyromonas gingivalis” published in Nature Microbiology (including researchers from the Faculty of Biochemistry, Biophysics and Biotechnology of the Jagiellonian University) presented their recent discoveries concerning the protein complex known as RagAB, which enables the transport of peptides inside P. gingivalis cells. It consists of a large channel (marked in yellow in the diagram below) leading through the outer membrane and a lid (marked in red), which opens and closes, granting or denying access to the channel. Additional safety is provided by a cork (marked in blue) that blocks the channel entry, preventing the intake of unwelcome substances. When the complex is bound to a peptide, the cork becomes loosened by TonB protein located inside the bacterium, and the peptide can sneak inside and become ingested. It molecules of other substances (such as sugars, lipids, or antibiotics) tried to enter this structure, the cork would remain in place, blocking their way.
After the proteins are cut into pieces, the resultant peptides vary in contents and arrangement of protein-building amino acids (shown as purple balls). The abovementioned article also contains a detailed description of P. gingivalis preferences regarding the contents and length of the absorbed peptides.
Perspectives for the future
RagAB complex has been the first described system of peptide transport in Gram-negative bacteria, which include P. gingivalis along with lots of other bacteria species, many of which are pathogens. The detailed description of the structure of this complex will enable scientists not only to better understand the functioning of Gram-negative bacteria, but also to design drugs preventing the absorption of peptides by the complex, hindering the growth of these pathogens.
Original text by Dr Mariusz Madej from the Department of Microbiology at the JU Faculty of Biochemistry, Biophysics and Biotechnology: www.nauka.uj.edu.pl
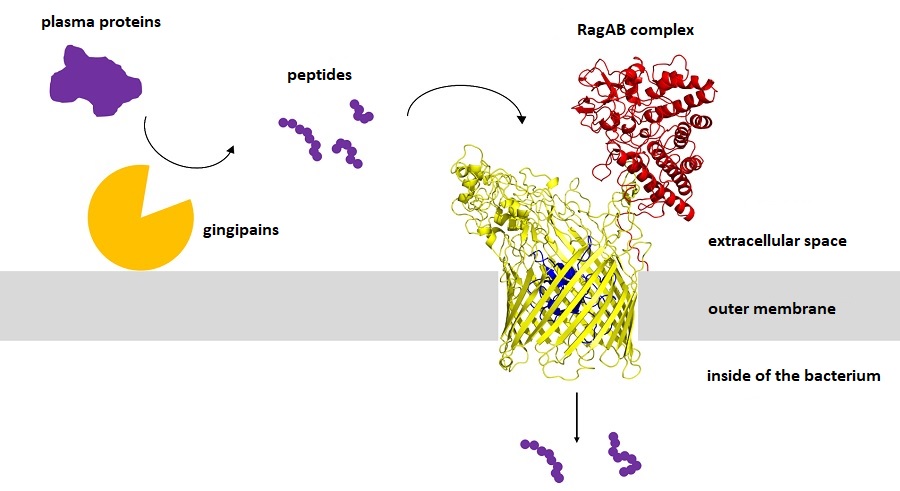
A diagram showing RagAB protein complex: yellow – the channel leading through the outer membrane; red – the lid that opens or closes access to the channel; blue - the cork blocking the channel entry, preventing the intake of unwelcome substances; purple – peptides. Image by Mariusz Madej